Participants sought for hearing loss drug trial
 People with hearing loss are being invited to join a clinical trial of a new drug call ed REGAIN.
People with hearing loss are being invited to join a clinical trial of a new drug call ed REGAIN.
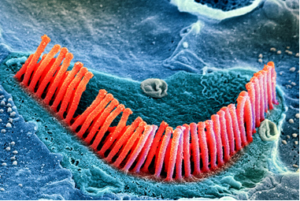
Age-related hearing loss is usually caused by loss of inner ear sensory cells. The assumption has long been that this type of hearing loss (“sensorineural hearing loss”) is irreversible because once the hair cells in the inner ear (“cochlea”) become damaged or are lost, they cannot regenerate.
However, recent studies in animals with hearing loss have shown that new and functioning inner ear hair cells can be generated using a drug, called a Gamma Secretase Inhibitor, that is locally applied to the ear.
The REGAIN trial tests if it is safe to use this drug in patients with hearing loss and if it improves their hearing.
The safety part of the trial is currently being carried out at The Royal National Ear, Nose and Throat Hospital in London (5 min walk from Kings Cross Station) by a team of expert clinicians and researchers under the lead of ENT surgeons Professor Anne Schilder and Professor Shakeel Saeed. Enrolment of eligible participants is happening on an ongoing basis.
The REGAIN team are looking for people aged 18-80 years with mild to moderate sensorineural hearing loss of less than 10 years duration, who are either using or have been previously offered hearing aids, to take part in this safety study.
If you suffer from sensorineural hearing loss and would like to take part, please contact the REGAIN study team using the details provided below:
By telephone: 020 3108 9344
By email: ei-regain@ucl.ac.uk
REGAIN website www.regainyourhearing.eu